Topic highlight
Focus Story: A brief literature focus on a hot state-of-the-art research area
Contributions and discussions
Metal-NHC Dynamics in Organometallic Chemistry and Catalysis
Valentine P. Ananikov
Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences
Leninsky Prospekt 47, Moscow, Russia; http://AnanikovLab.ru
N-heterocyclic carbenes (NHCs) are superior ligands for binding with transition metals and forming unique structures. High stability on air, stable metal-ligand framework and outstanding possibility to tune electronic and steric properties (by varying types of NHC rings and substituents) has led to a large variety of metal complexes with numerous applications in chemistry and catalysis. Applications of Metal/NHC (M/NHC) complexes in homogeneous catalysis are or particular importance, where a paramount progress was demonstrated in recent decades [1].
Detailed mechanistic studies have revealed a unique picture with dual opportunities in catalysis: i) homogeneous catalysis with molecular M/NHC complexes, and ii) nanoparticle catalysis with NHC-stabilized metal clusters. The interchange between two types of catalysis was first described by our group upon studying a well-known Mizoroki-Heck reaction [2]. A new mode of catalysis, which takes an advantage of chemical lability of the M-NHC bond (rather than stability, as it was previously assumed), was revealed in the experiment [2]. Facile R-NHC coupling was shown to be a general process for various metal complexes and organic groups R [3],[4],[5],[6], thus opening excellent possibilities for in situ generation of stabilized metal clusters and nanoparticles from M/NHC precursors. A possibility of the reverse process (R-NHC oxidative addition) and NHC-mediated leaching of metal species from the surface of nanoparticles [7] give rise to construction of dynamic catalytic systems.
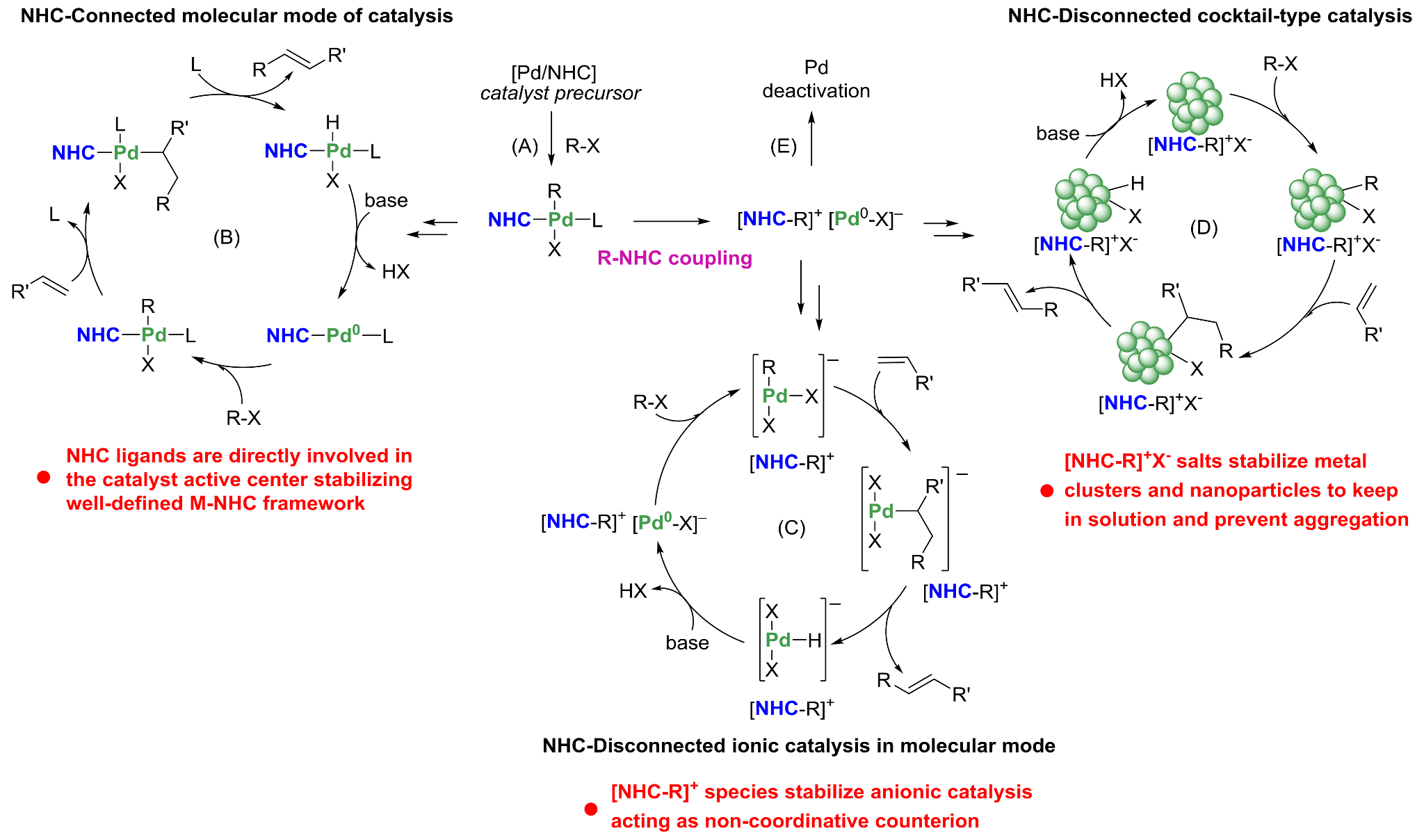
Figure 1. Universal range of catalytic systems accessible from M/NHC complexes (image from ref. [8], (C) The Royal Society of Chemistry, CC-BY-NC license).
Detailed analysis of the literature has shown that M-NHC dynamics indeed may be present in a number of systems for various metals and NHC ligands [8]. Using a single M/NHC complex three types of catalytic systems may be generated: i) molecular catalysis, ii) nanoparticle catalysis, and iii) ionic catalysis (Figure 1) [8]. It appears that many M/NHC-catalyzed reactions may involve dynamic and cocktail-type systems, with dynamic interconversion of active species during the reaction.
Interestingly, NHC ligands continue to play an important role in the stabilization of metal centers after breakage of the M-NHC bond. Stabilization of metal clusters and nanoparticles via azolium species or stabilization of molecular ionic complexes via counterion interactions were considered (Figure 1) [8].
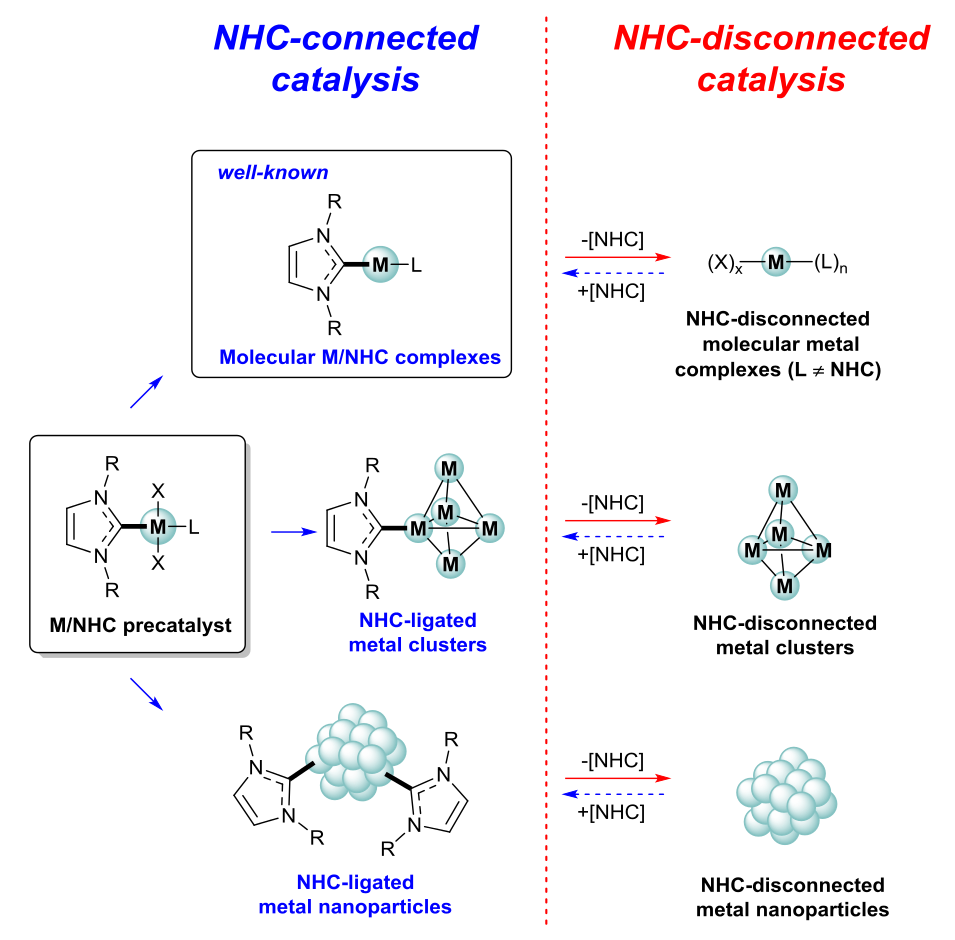
Figure 2. Single M/NHC complex as a universal precursor for a number of tuned catalytic systems (image from ref. [8], (C) The Royal Society of Chemistry, CC-BY-NC license).
Intrinsic dynamic behavior recalls for more studies on the synthesis, structure, stability and reactivity of M/NHC complexes in organometallic chemistry. New wave of dynamic and cocktail-type catalysts design based on the M/NHC complexes is anticipated in the near future for development of a new generation of catalytic systems. Formulation of the concept of “NHC-connected” and “NHC-disconnected” modes of catalysis (Figure 2) [8] is of much importance to develop efficient and recyclable catalytic systems. Thus, understanding the role of NHC ligands and controlling the nature of catalytic centers becomes the topic of key importance.
References
[1] (a) . Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988-10031. [DOI]
(b) S. Budagumpi, R. S. Keri, G. Achar, and K. N. Brinda. Coinage Metal Complexes of Chiral N‐Heterocyclic Carbene Ligands: Syntheses and Applications in Asymmetric Catalysis. Adv. Synth. Catal. 2020 , 362 , 970‑997. [DOI]
(c) W.A. Herrmann. N‐Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290-1309. [DOI]
(d) E. Kantchev, C. O'Brien, and M. Organ. Palladium Complexes of N-Heterocyclic Carbenes as Catalysts for Cross-Coupling Reactions—A Synthetic Chemist's Perspective. Angew. Chem. Int. Ed. 2007, 46, 2768-2813. [DOI]
(e) C. Fliedel, A. Labande, E. Manoury, and
R. Poli. Chiral N-heterocyclic carbene ligands with additional chelating
group(s) applied to homogeneous metal-mediated asymmetric catalysis.
Coord. Chem. Rev. 2019, 394, 65-103. [DOI]
(f) R.D.J. Froese, C. Lombardi, M. Pompeo, R.P. Rucker, and M. Organ.l Designing Pd–N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [DOI]
(g) T. Simler, and P. Braunstein. N-Heterocyclic Carbene Complexes of Copper, Nickel, and Cobalt. Chem. Rev. 2019, 119, 3730–3961. [DOI]
(h) D. Janssen-Müller, C. Schlepphorst, and F. Glorius. Privileged chiral N-heterocyclic carbene ligands for asymmetric transition-metal catalysis. Chem. Soc. Rev. 2017, 46, 4845-4854. [DOI]
(i) G.C. Fortman, and S.P. Nolan. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: a perfect union. Chem. Soc. Rev. 2011, 40, 5151-5169. [DOI]
(j) V. Nesterov, D. Reiter, P. Bag, P. Frisch, R. Holzner, A. Porzelt, and S. Inoue. NHCs in Main Group Chemistry. Chem. Rev. 2018, 118, 9678–9842. [DOI]
(k) T. Dröge, and F. Glorius. The Measure of All Rings—N‐Heterocyclic Carbenes. Angew. Chem. Int. Ed. 2010, 49, 6940-6952. [DOI]
[2] A.V. Astakhov, O.V. Khazipov, A.Yu. Chernenko, D.V. Pasyukov, A.S. Kashin, E.G. Gordeev, V.N. Khrustalev, V.M. Chernyshev, and V.P. Ananikov. A New Mode of Operation of Pd-NHC Systems Studied in a Catalytic Mizoroki–Heck Reaction. Organometallics 2017, 36, 1981-1992. [DOI]
[3] E.G. Gordeev, D.B. Eremin, V.M. Chernyshev, and V.P. Ananikov. Influence of R–NHC Coupling on the Outcome of R–X Oxidative Addition to Pd/NHC Complexes (R = Me, Ph, Vinyl, Ethynyl). Organometallics 2018, 37, 787-796. [DOI]
[4] O.V. Khazipov, M.A. Shevchenko, A.Yu. Chernenko, A.V. Astakhov, D.V. Pasyukov, D.B. Eremin, Y.V. Zubavichus, V.N. Khrustalev, V.M. Chernyshev, and V.P. Ananikov. Fast and Slow Release of Catalytically Active Species in Metal/NHC Systems Induced by Aliphatic Amines. Organometallics 2018, 37, 1483-1492. [DOI]
[5] A.V. Astakhov, S. Soliev, E.G. Gordeev, V.M. Chernyshev and V. P. Ananikov. Relative Stability of M/NHC Complexes (M = Ni, Pd, Pt) against R−NHC, X−NHC and X−X Couplings in M(0)/M(II) and M(II)/M(IV) Catalytic Cycles: a Theoretical Study. Dalton Trans., 2019, 48, 17052-17062. [DOI]
[6] D.B. Eremin, E.A. Denisova, A.Yu. Kostyukovich, J. Martens, G. Berden, J. Oomens, V.N. Khrustalev, V. M. Chernyshev, and V. P. Ananikov, Chem. ‒ Eur. J. 2019, 25, 16564. [DOI]
[7] E.A. Denisova, D.B. Eremin, E.G. Gordeev, A.M. Tsedilin, and V.P. Ananikov. Addressing Reversibility of R–NHC Coupling on Palladium: Is Nano-to-Molecular Transition Possible for the Pd/NHC System? Inorg. Chem. 2019, 58, 12218-12227. [DOI]
[8] V.M. Chernyshev, E.A. Denisova, D.B. Eremin, and V.P. Ananikov. The key role of R–NHC coupling (R = C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species. Chem. Sci., 2020, 11, 6957-6977. [DOI]